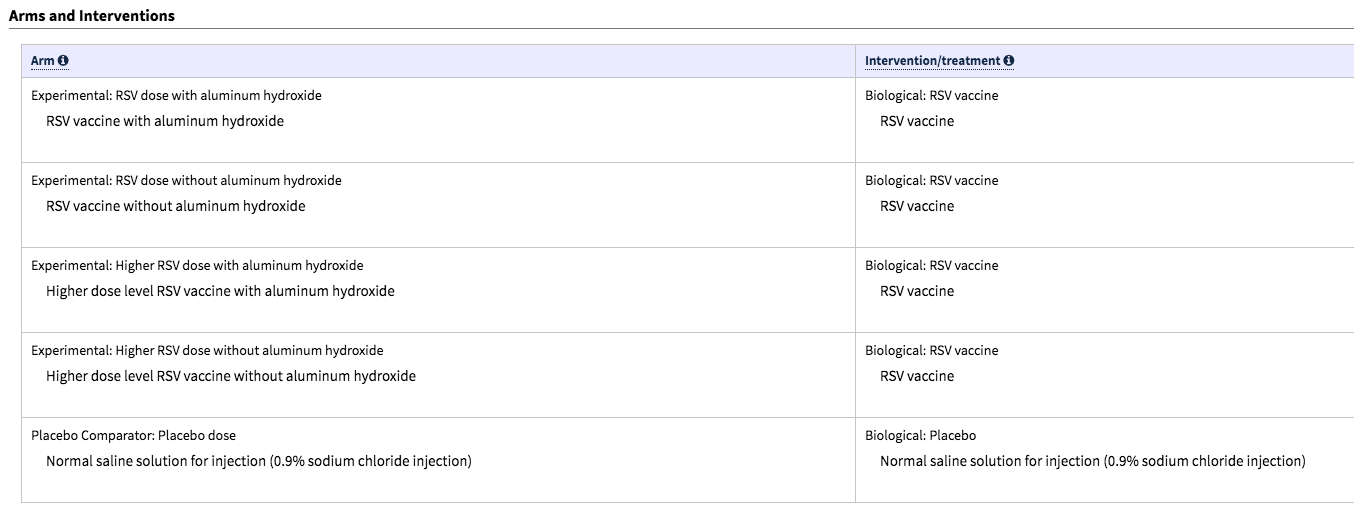
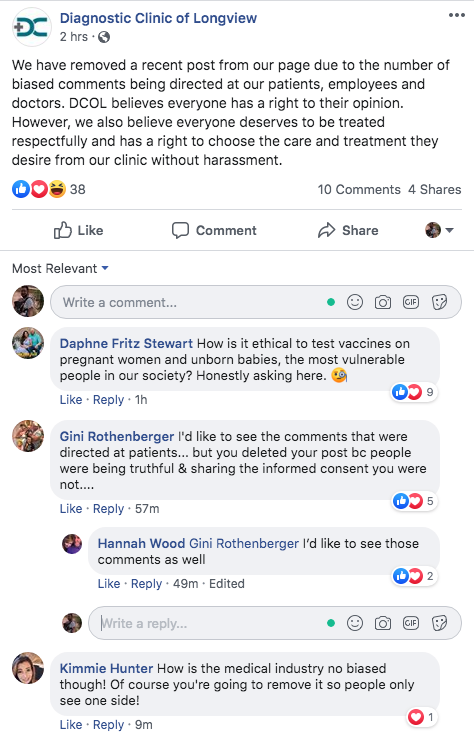
PFIZER TESTING VACCINES ON HEALTHY PREGNANT WOMEN IN NATIONWIDE TRIAL
“Why in the world would anyone allow their unborn child to be a test subject?!”
BY KELEN MCBREEN
SEE: https://www.infowars.com/pfizer-testing-vaccines-on-healthy-pregnant-women-in-nationwide-trial/;
republished below in full unedited for informational, educational and research purposes:
Del Bigtree joins David Knight to discuss the flu vaccine and the myths behind it.He gets into lawsuits that have been swept under the radar, as well gives information on how vaccines have been linked to autism.